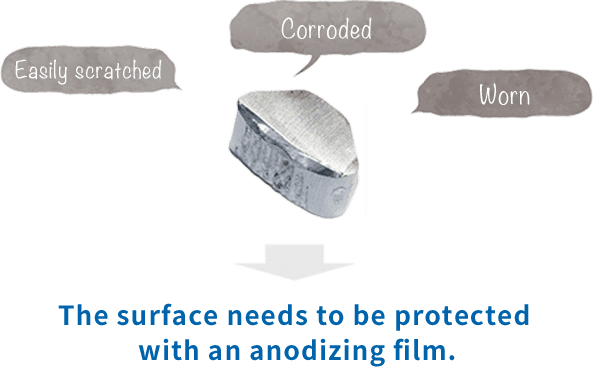
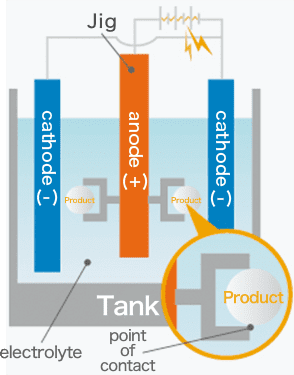
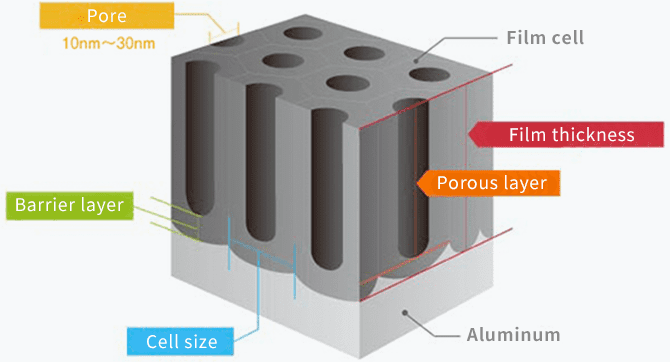
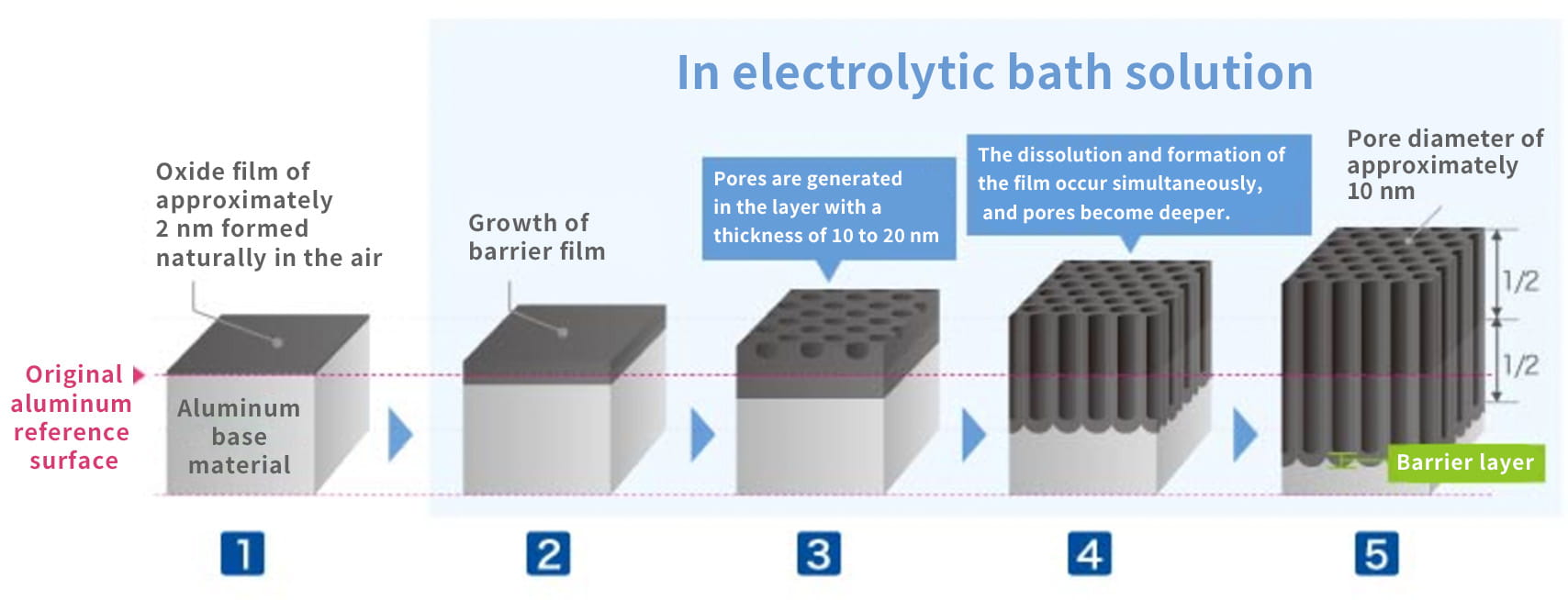
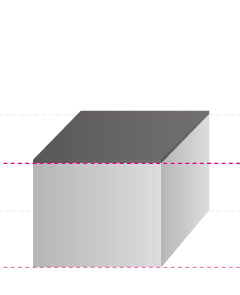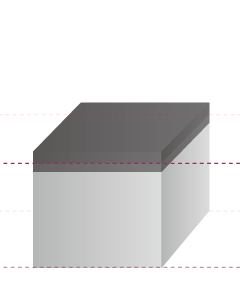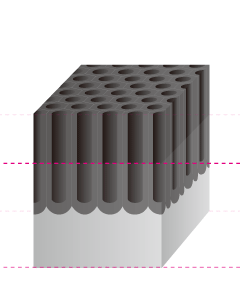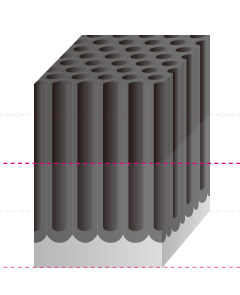
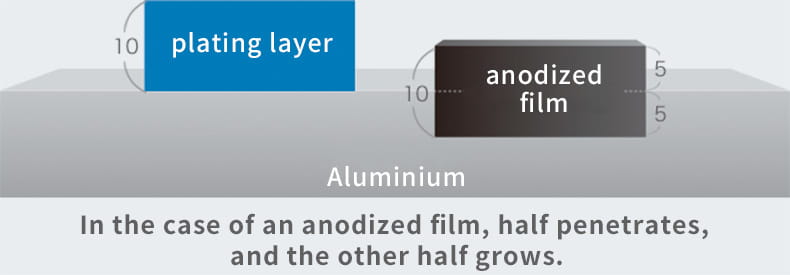
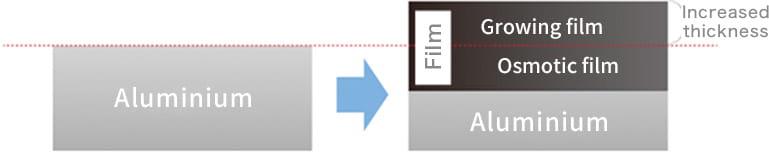
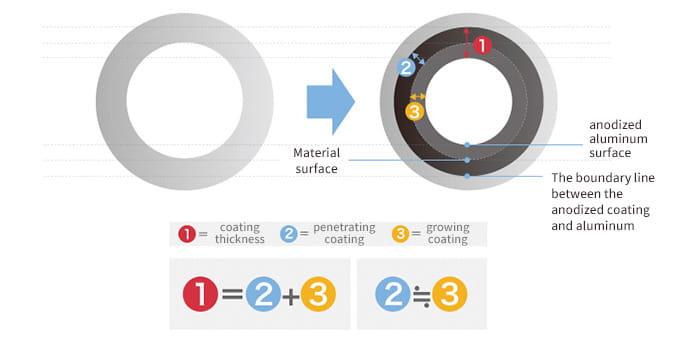
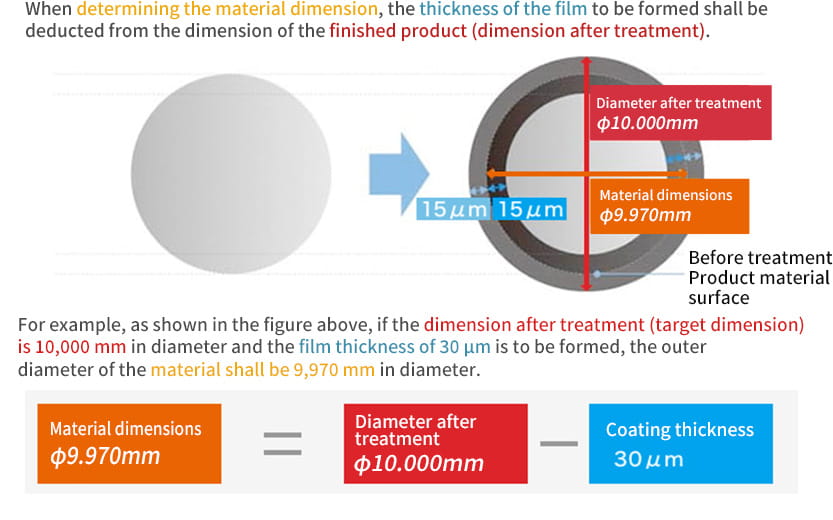
In fact, anodizing and plating are totally different, although they are bracketed together as metallic surface treatments. In the case of anodizing, films grow upward from the aluminum surface (standard surface) as the growing coating and downward from there as the penetrating coating. Because they grow uniformly, if the original aluminum surface has bumps and dips, they are anodized as they are.
Anodizing does not have the effect of smoothing the surface like plating. In addition, if a material that has already been anodized is anodized again, the material thickness decreases.
It is because the whole Osmotic film needs to be removed chemically before re-anodizing.
On the other hand, in the case of plating, other metals are put on an object to be plated in turn.
Therefore, the theory is completely different.