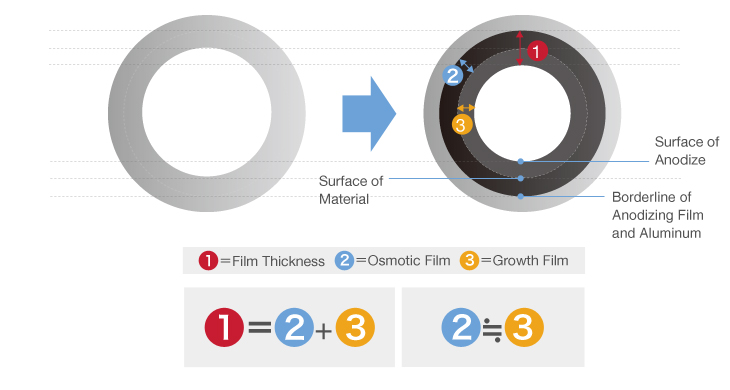
Anodizing is a surface treatment process in which aluminum (anode) is electrolyzed to artificially generate an oxide film (rust).
Aluminum easily reacts with oxygen, creating an extremely thin oxide film when in contact with air.
This naturally produced film forms a protective layer that prevents rust, resulting in aluminum’s characteristically good corrosion resistance.
However, this film is extremely thin and can corrode due to environmentally-induced chemical reactions, necessitating a protective surface treatment. That is where anodizing comes in.
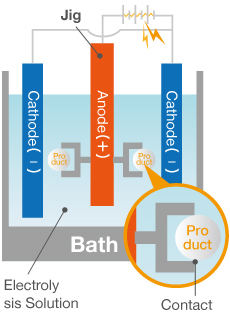
1
The jig holding the aluminum is dipped in the electrolysis solution (sulfuric acid or oxalic acid).
2
Connect electrode to jig and add plus current while simultaneously adding negative current to the cathode.
3
Electrolysis generates an oxide film (aluminum oxide) on the surface of the aluminum.
The term pore refers to the holes in the oxide film that are 100Å-300Å(Angstrom) in diameter. There are actually an amazing 5-70 billion of them per cubic centimeter. The entire population of the world could easily fit in this small space if shrunk down to the atomic level.
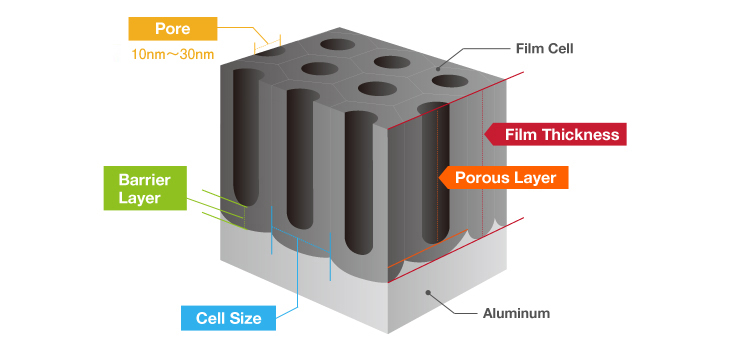
Electrical induction of aluminum melts the surface, simultaneously generating osmotic film and oxide film to form 3-dimensional cells over time. It may be helpful to think of the basic structure as a bunch of hexagonal pencils sprouting from the surface of the aluminum.
1
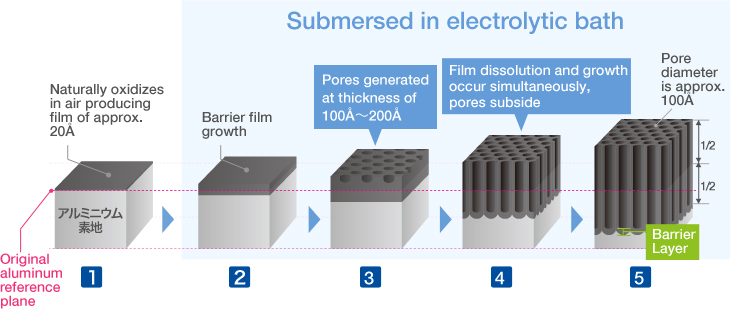
Aluminum is an active metal that naturally reacts in air to form an oxide film of approx. 20Å thick.
2
Aluminum submersed in the electrolytic bath will oxidize and generate an oxide film.
3
In order to facilitate better electrolyzation, sulfate ion penetrates depressions on the film’s surface, resulting in a localized elution reaction that creates aluminum sulfate, opening countless pores on the surface.
4
The oxidative reaction and elution reaction progress simultaneously at the base of the pores, creation a regular pore structure.
5
Film thickness is proportional to electrolysis time.


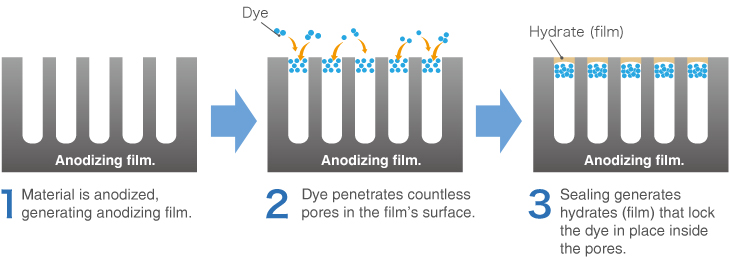
Dye penetrates the pores generated in the anodizing coating (anodizing), sealing the surface to create a colored surface that will remain until the oxide film itself wears away.
■Color Anodizing
If each pore in the oxide film is filled with red dye, for example, then red anodizing will be produced. The product of this dyeing process is called color anodizing.
■Natural Coloring
The product’s color can be altered by simply changing the dye used on the oxide film. Another method called natural coloring can be employed to alter the color of the actual surface of special aluminum alloys.
| Standard Anodizing (white / color) | Hard Anodizing | |
|---|---|---|
| Treatment Overview | Most common treatment method using sulfuric acid bath | Treatment in low temperature electrolytic bath generates thick, hard film |
| Color Tone | Usually white in color, but dyeing can be used to produce a specified color | Naturally has grayish color that will differ with the type of aluminum and film thickness |
| Hardness | Approx. 200HV Aluminum < Standard Anodizing < Iron |
Greater than. 400HV Iron(non-heat treated) < hard anodizing |
| Film Thickness | Decided by application conditions, generally around 5μ-25μ | Generally specified 2.0μ-7.0μ based on wear resistance, electric insulation properties |
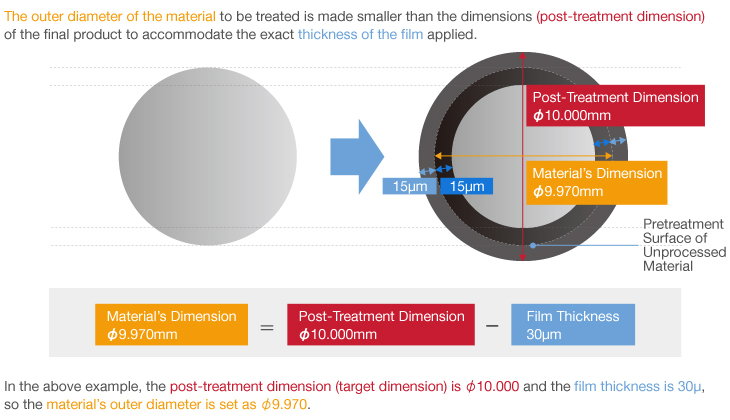
| Dimensions | Plus 1/2 film thickness » Click here for details | Plus 1/2 film thickness » Click here for details |
| Main Applications | Construction materials, industrial goods, household goods, ornaments |
Sliding parts including shafts and rollers, aircraft parts |
| Standard Anodizing (white / color) | |
|---|---|
| Treatment Overview | Most common treatment method using sulfuric acid bath |
| Color Tone | Usually white in color, but dyeing can be used to produce a specified color |
| Hardness | Approx. 200HV Aluminum < Standard Anodizing < Iron |
| Film Thickness | Decided by application conditions, generally around 5μ-25μ |
| Dimensions | Plus 1/2 film thickness » Click here for details |
| Main Applications | Construction materials, industrial goods, household goods, ornaments |
| Hard Anodizing | |
|---|---|
| Treatment Overview | Treatment in low temperature electrolytic bath generates thick, hard film |
| Color Tone | Naturally has grayish color that will differ with the type of aluminum and film thickness |
| Hardness | Greater than. 400HV Iron(non-heat treated) < hard anodizing |
| Film Thickness | Generally specified 2.0μ-7.0μ based on wear resistance, electric insulation properties |
| Dimensions | Plus 1/2 film thickness » Click here for details |
| Main Applications | Sliding parts including shafts and rollers, aircraft parts |
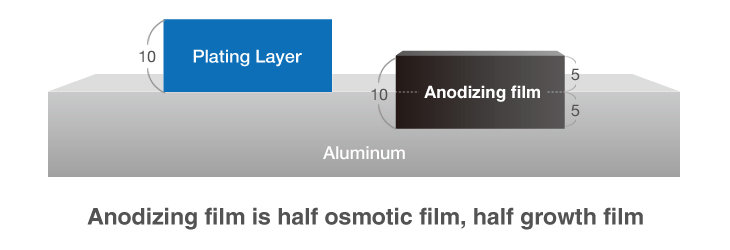
Anodizing and Plating are Completely Different Products
While anodizing and plating are often regarded as similar in that they both involve the surface treatment of metal, they are in fact completely different products.
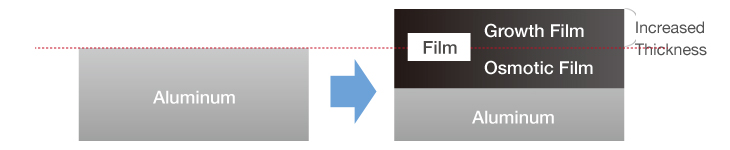
Anodizing generates both a growth film on the surface, and an osmotic film below the surface of aluminum. The two films grow uniformly, meaning that any surface unevenness will result in anodizing with the same imperfections.
This fails to create a smooth surface finish akin to proper coating.
Furthermore, the reapplication of anodizing to a surface already treated with hard anodizing will result in thinning as the osmotic film has to be chemically stripped before reapplication.
Plating, however, is a completely different concept in that it involves the sequential application of metal to the target’s surface.